Abstract
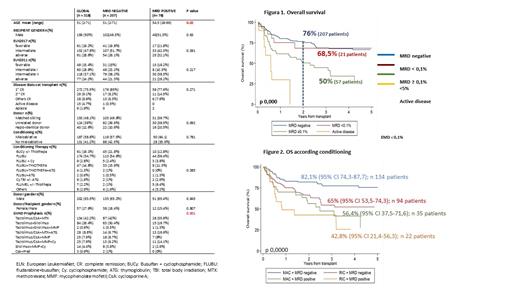
Introduction: Acute myeloblastic leukaemia (AML) is an heterogeneous disease with different molecular and prognostic characteristics. According to the comorbidities and the revised 2017 European Leukaemia genetic risk stratification (ELN17), allogeneic hematopoietic cell transplantation (HCT) is the best therapeutic option for many patients with AML (Grimm, Blood Adv 2020). However, relapse remains the main cause of mortality after transplantation. Impact of MRD on the outcome of patients is well recognized and ELN2017 introduced the new response category complete remission (CR) without MRD (Döhner H, Blood 2017). Detection of measurable residual disease (MRD) by multiparameter flow cytometry (MFC) in AML before allogeneic HCT could be a powerful predictor of outcome and decisive when establishing strategies that modify the prognosis of these patients. Methods: Retrospective multicentre analysis of MRD by MFC of patients undergoing transplantation allogeneic in 4 centres during the period from 2012 to 2020. Both Leukaemia Associated Aberrant Immunophenotype (LAIP) and different from normal (DFN) approach were used to analyse the MRD. The MRD was carried out with 8-color panels based on Euroflow protocols. The samples were acquired in 8-color digital cytometers (FACSCanto II) calibrated and compensated according to Euroflow protocols. Results: 295 of 318 patients were evaluated. Table 1 shows the characteristics of the patients. 285 (96.7%) were in complete remission (CR), 207 had negative MRD, in 21 MRD was less than 0.1% (MRD-low) and in 57 greater than or equal to 0.1% (MRD-high). At 2 years, the overall survival (OS) and leukaemia-free survival (LFS) in the whole group were 69% (95% CI 63.18-74.18) and 58.4% (95% CI 52.4-63.9) respectively. In CR patients, MRD levels significantly influenced on outcomes, with OS and LFS of 76.7% and 67.6% for negative MRD, 68.5% and 49.7% MRD-low and 50 % and 36.6% in MRD-high, p <0.001) (Figure 1). Considering only MRD-high as positive, according to ELN17, cumulative incidence of relapse (CIR) at 2 and 5 years were significantly lower among those with positive MRD: 22% (95% CI 17-28.1%) and 27% (95% CI 21%-33.5%) for negative MRD vs 46,5% (95% CI 32.4%-59.5%) and 50% (95% CI 34.8%-63.2%) in positive MRD, p 0.0005. No differences were observed in terms of non relapse mortality (p 0.2).. Likewise, positive MRD also identified different prognostic subgroups within the ELN2017 subgroups: OS and LFS among high-risk ELN2017 patients of 63.6% and 52.3% in negative MRD vs 35.7% and 18.2% in positive MRD patients, p = 0.0085 and p = 0.0094, respectively; for intermediate risk: 77% and 67.6% in negative MRD vs 67% and 50.5% in positive MRD patients, p = 0.23 and p = 0.056; and for favourable: 84% and 77.7% in negative MRD vs 48% and 39.2% in patients with positive MRD, p = 0.0051 and p = 0.0341. Considering the conditioning regimen, patients with MRD negative before transplant had better OS and LFS at 2 years (82% and 71.4% among those received myeloablative conditioning and 65% and 57.6% among those who received reduced intensity, respectively) than those who had positive MRD prior to transplant (56% and 44.4% in myeloablative and 43% and 25.5% in reduced intensity) (p <0.001) (Figure 2). In multivariate time-dependent analysis, age (HR 1.019 p = 0.024-95% CI 1.001-1,038), adverse risk group according to ELN17 (HR 2.13 p = 0.033 CI95 1.54-3.93 ) and MRD before transplant (HR 3.8 p <0.001 95 CI 1.55-3.93) significantly influenced survival. Conclusions: Detection of MRD prior to transplant by MFC identifies a group of patients with a worse prognosis and could be key when selecting the most appropriate therapeutic strategy.
Caballero: Celgene: Consultancy. Belén Vidriales: Roche: Consultancy; Novartis: Speakers Bureau; Jazz: Consultancy, Speakers Bureau; Astellas: Consultancy, Speakers Bureau. Montesinos: Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Stemline/Menarini: Consultancy; Tolero Pharmaceutical: Consultancy; Agios: Consultancy; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Forma Therapeutics: Consultancy; Glycomimetics: Consultancy; Teva: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Astellas Pharma, Inc.: Consultancy, Honoraria, Other: Advisory board, Research Funding, Speakers Bureau. Perez-Simon: JANSSEN, TAKEDA, PFIZER, JAZZ, BMS, AMGEN, GILEAD: Other: honorarium or budget for research projects and/or participation in advisory boards and / or learning activities and / or conferences.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal